First-ever injectable HIV prevention drug approved by FDA

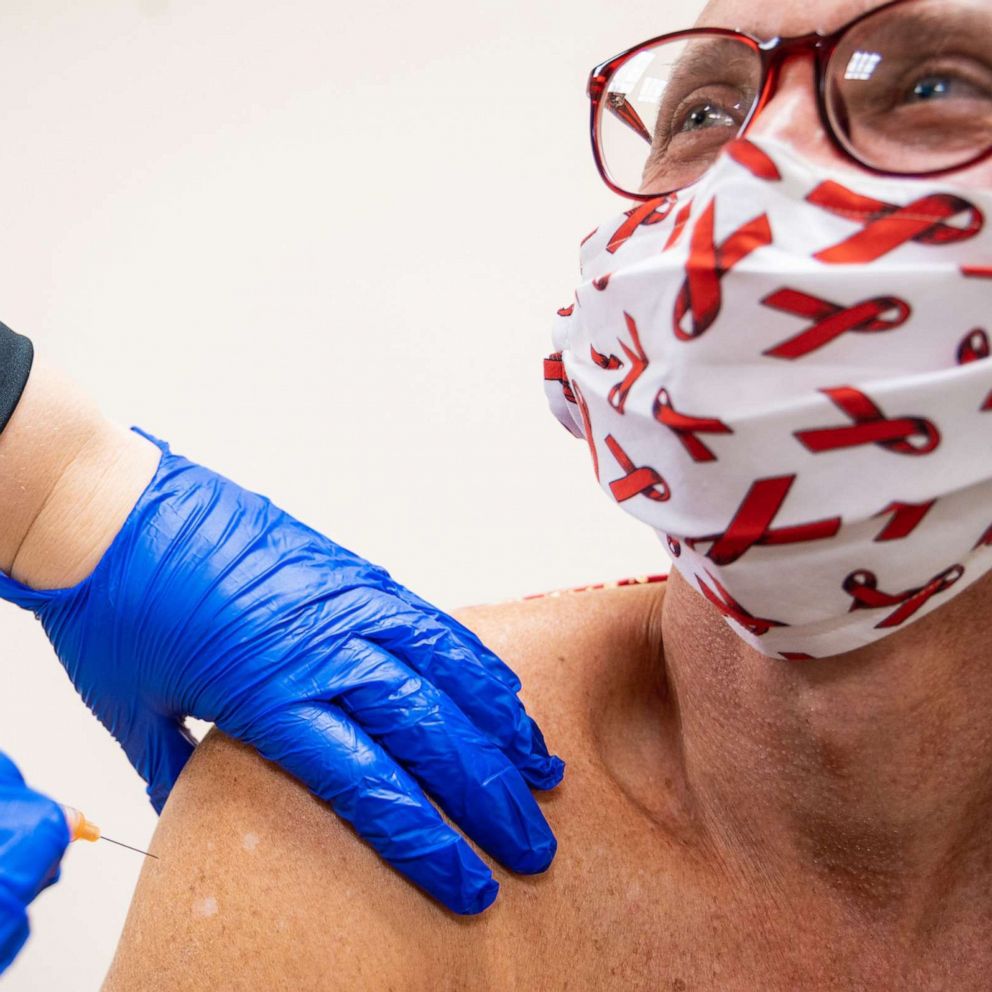
The U.S. Food and Drug Administration (FDA) has approved the first-ever long-acting injectable drug for HIV prevention.
Until this week, the only FDA-licensed and approved medications for HIV pre-exposure prophylaxis, most commonly known as PrEP, were daily oral pills containing the HIV treatment drugs tenofovir and emtricitabine, which slow the progression of an HIV infection in the body.
PrEP is taken daily so that it builds up in your system, to the point that if there is an HIV infection, it prevents the virus from replicating and spreading throughout the body.
When taken as prescribed, PrEP services reduce the risk of getting HIV from sex by about 99%, according to new data from the CDC. Now, individuals who feel at-risk of HIV infection have the option of taking the daily pill, or the new shot every two months, after two initiation injections administered one month apart.
"This injection, given every two months, will be critical to addressing the HIV epidemic in the U.S., including helping high-risk individuals and certain groups where adherence to daily medication has been a major challenge or not a realistic option," the FDA said in a statement.
According to Dr. Darien Sutton, Emergency Medicine Physician, in an interview with "Good Morning America," "This is a game-changer in the world of HIV prevention."
"Patients often have difficulty complying with any oral medication, so a bi-monthly injection can truly change the landscape in terms of HIV prevention. Having a bi-monthly treatment also serves as an opportunity to interact with a patient, share risk reduction sexual health education and complete necessary screenings."
"Patients on PrEP can often feel stigmatized with taking daily medication," he told "GMA." "Some have shared with me that they fear simple actions, like picking up their medications from the pharmacy due to fear of stigmatization. This stigma unfortunately doesn't stop at the pharmacy, as many also fear being seen carrying their preventative medications in public."
Sutton added, "The study was also inclusive, including transgender women, which allows better applicability with diverse patient populations."
CDC data shows that an estimated 34,800 people in the United States acquired HIV in 2019, the most recent year for which data are available.
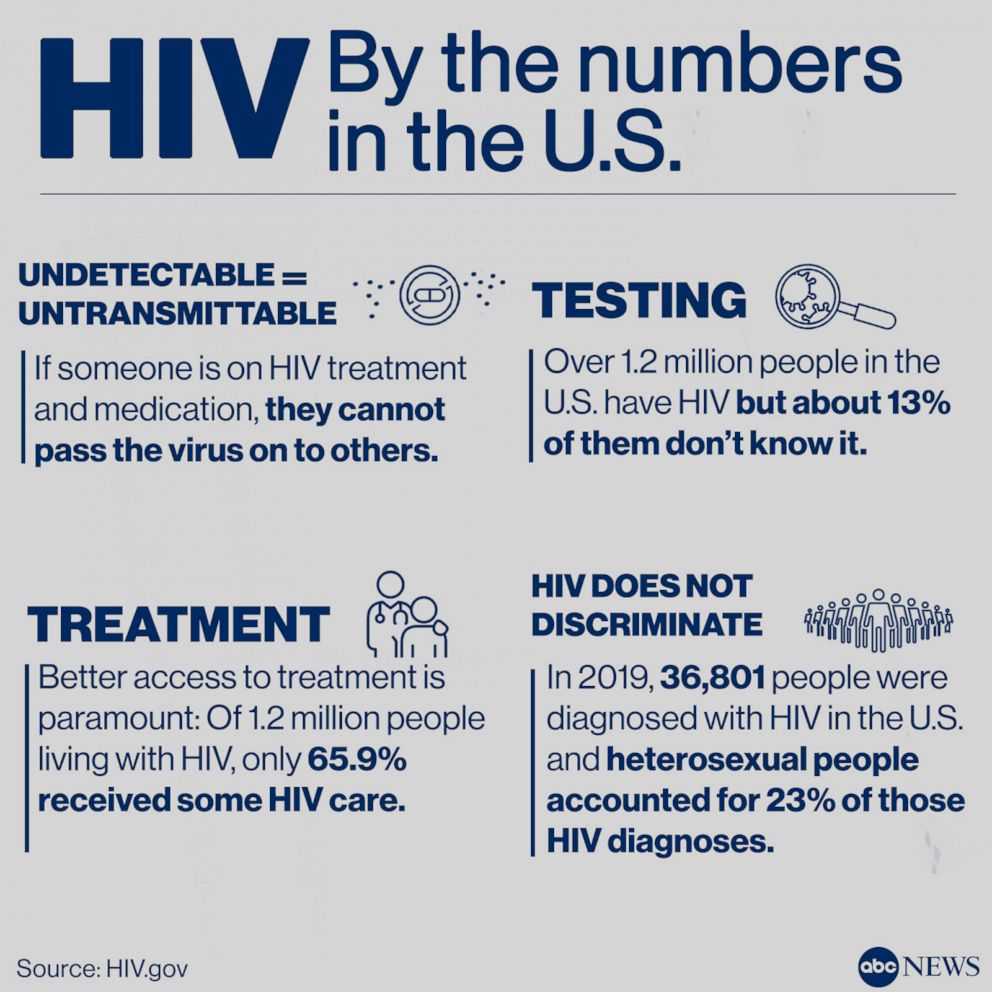
Men who have sex with men, transgender women who have sex with men, and Black cisgender women are among those disproportionately affected by HIV in the U.S.
Heterosexual people made up 23% of all HIV diagnoses in the U.S. and six dependent areas in 2019. Specifically, heterosexual men accounted for 7% of new HIV diagnoses and heterosexual women accounted for 16%.
The FDA approval comes on the heels of a CDC recommendation this month that there be an expansion of HIV prevention medication to close the gap on PrEP implementation.
In a release, the National Institute of Allergy and Infectious Diseases (NIHAID) also made a nod to the FDA approval, saying in part, "These medications are highly effective at preventing HIV when taken daily as prescribed, however, taking a pill daily while feeling healthy can be challenging." Adding, "Long-acting injectable cabotegravir PrEP is a less frequent, more discreet HIV prevention option that may be more desirable for some people."